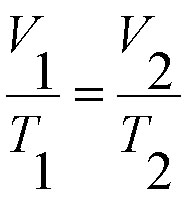Charles's law states: If the pressure is constant, the volume of a mass of gas is proportional to the absolute temperature. The absolute temperature is always 273 Kelvin more than the centigrade temperature.
A useful expression of this physics law is:
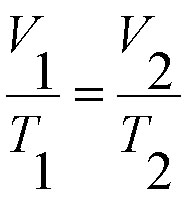 |
Where V1 is the volume of a mass of gas at temperature T1 kelvins and V2 is its volume after the temperature has changed to T2 kelvins. |
|
|
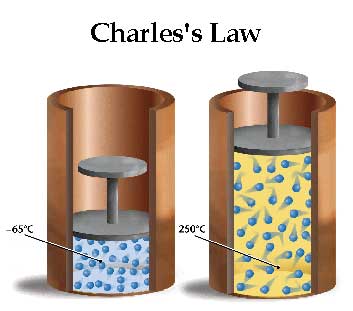
Another illustration that is helpful to understand Charles's law is when you have the same amount of molecules in two separate volumes, yet they have displace different volumes caused by 2 different temperatures. |
|
This graph plots the formula and will show either the volume or the temperature as they change. |
 |